Stage 2 dosing
TZIELD is a single-course treatment completed over 14 consecutive days.1


Recommended dosing schedule1
TZIELD is administered by intravenous infusion over 14 consecutive days.
Day
Dose mcg/m2
1
65
2
125
3
250
4
500
5-14
1030
Administered at patients’ preference—TZIELD can be given at an infusion center, a patient’s home, or a combination of both.
Dosing and administration information1

2 mg per 2 mL (1 mg/mL), clear and colorless solution in a single-dose vial.

Resume by administering all remaining doses on consecutive days to complete the 14-day course.
Determine the dose1
TZIELD is administered by intravenous infusion over a minimum of 30 minutes using BSA-based dosing.
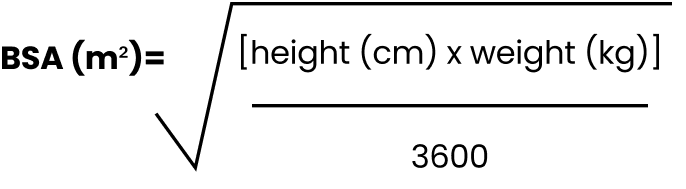
Calculate BSA dosing with the Mosteller formula2
BSA EQUATION

EXAMPLE
Male, 8 years old, 120 cm, 26 kg

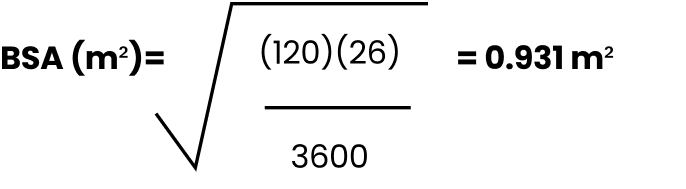
When calculating BSA, round to the 100th using standard rounding rules (example, 0.93 m2).1
Based on BSA dosing requirements, 2 vials may be needed for some individuals (BSA >1.94 m2) for Days 5-14.1
BSA=body surface area.
Watch step-by-step TZIELD dosing instructions for Stage 2
react player not avalible serverside
TZIELD Dosing and Administration Video Transcript
NARRATOR: Indication. TZIELD (TEE-zeeld) teplizumab-mzwv (tep-LIH-zoo-mab-M-Z-W-V) is a CD3-directed monoclonal antibody (mon-oh-KLO-nal AN-tee-bah-dee) indicated to delay the onset of Stage 3 type 1 diabetes (or T1D) in adults and pediatric patients aged 8 years and older with Stage 2 T1D.
Important Safety Information. Warnings and Precautions.Cytokine (SY-toh-kyn) Release Syndrome (CRS): CRS occurred in TZIELD-treated patients during the treatment period and through 28 days after the last drug administration. Prior to TZIELD treatment, premedicate with antipyretics (an-tee-py-REH-tiks), antihistamines (an-tee-HIS-tah-mins) and/or antiemetics (an-tee-eh-MI-tiks), and treat similarly if symptoms occur during treatment. If severe CRS develops, consider pausing dosing for 1 day to 2 days and administering the remaining doses to complete the full 14-day course on consecutive days; or discontinue treatment. Monitor liver enzymes during treatment. Discontinue TZIELD treatment in patients who develop elevated alanine aminotransferase (AL-uh-neen ah-MEE-no-TRANS-fur-ays) or aspartate aminotransferase (ah-SPAHR-tayt ah-MEE-no-TRANS-fur-ays) more than 5 times the upper limit of normal or bilirubin (bih-lih-ROO-bin) more than 3 times ULN.
NARRATOR: Serious Infections: Use of TZIELD is not recommended in patients with active serious infection or chronic infection other than localized skin infections. Monitor patients for signs and symptoms of infection during and after TZIELD administration. If serious infection develops, treat appropriately, and discontinue TZIELD. Lymphopenia (lim-foh-PEE-nee-ah): Lymphopenia occurred in most TZIELD-treated patients. For most patients, lymphocyte (LIM-fuh-site) levels began to recover after the fifth day of treatment and returned to pretreatment values within two weeks after treatment completion and without dose interruption. Monitor white blood cell counts during the treatment period. If prolonged severe lymphopenia develops (less than 500 cells per microliter lasting 1 week or longer), discontinue TZIELD. Please see additional Important Safety Information throughout and accompanying full Prescribing Information or go to TZIELDhcp.com.
NARRATOR: Stage 2 T1D should be confirmed by documenting at least two positive pancreatic islet (pan-kree-AT-ik EYE-let) cell autoantibodies in those who have dysglycemia (dis-gly-SEEM-ee-ah) without overt hyperglycemia (hy-per-gly-SEEM-ee-ah) using an oral glucose tolerance test (or OGTT) or alternative method if appropriate and OGTT is not available. Ensure the clinical history of the patient does not suggest type 2 diabetes.
NARRATOR: TZIELD should only be administered by trained healthcare professionals. This is an instructional video for demonstration purposes only and is not intended to show proper aseptic technique. Follow your institution’s guidelines for proper aseptic technique. The information in this video does not constitute a recommendation as to the appropriate use of TZIELD for a particular patient. Providers should use their clinical judgment while administering TZIELD. Please see Important Safety Information throughout this video, and please read the Prescribing Information prior to administration.
NURSE: Hi there. Today, we’ll show you how to administer TZIELD, a CD3-directed monoclonal antibody (mon-oh-KLO-nal AN-tee-bah-dee) administered via IV (I-V) infusion. In this video, we’ll cover the dosing, preparation, and administration instructions for infusing TZIELD.
NARRATOR: TZIELD is administered by intravenous infusion (over a minimum of 30 minutes), using a body surface area, or BSA (B-S-A)-based dosing, once daily for 14 consecutive days.
NARRATOR: Laboratory Evaluation and Vaccination Prior to Initiation. Prior to initiating TZIELD, obtain a complete blood count and liver enzyme tests. Use of TZIELD is not recommended in patients with certain laboratory abnormalities. Use of TZIELD is NOT recommended in patients with: lymphocyte count less than one thousand lymphocytes per microliter, hemoglobin (HEE-muh-glow-bin) less than 10 grams per deciliter, platelet count less than one hundred thousand platelets per microliter, absolute neutrophil (NOO-troh-fil) count less than one thousand neutrophils per microliter, elevated ALT or AST greater than 2 times the upper limit of normal or bilirubin greater than one point five times the upper limit of normal, laboratory or clinical evidence of acute infection with Epstein-Barr (EHP-styn-bahr) virus or cytomegalovirus (sy-toh-MEH-gah-low-vy-ruhs), active serious infection, or chronic active infection other than localized skin infections.
NARRATOR: Ensure that all age-appropriate vaccinations have been administered prior to starting TZIELD. Administer live-attenuated, or live, vaccines at least 8 weeks prior to treatment. Administer inactivated, or killed, vaccines or mRNA (M-R-N-A) vaccines at least 2 weeks prior to treatment.
NARRATOR: TZIELD must be diluted before infusion. The infusion solution is prepared with TZIELD, with the quantity depending on the patient’s BSA.
NARRATOR: The BSA (in square meters) can be calculated using the Mosteller (MOH-stel-ur) formula and will be based on the patient’s height and weight that is measured on the first day of treatment. When calculating BSA, round to the nearest 100th (2 decimal places) using standard rounding rules.
NARRATOR: TZIELD should be administered once daily for 14 consecutive days. The dose increases each day from days 1 through 5, then remains the same on days 5 through 14.NARRATOR: On day 1, administer 65 micrograms per meter squared. To calculate the daily infusion dose, multiply the patient’s BSA times 65 micrograms per meter squared.
NARRATOR: On day 2, administer one hundred twenty-five micrograms per meter squared. To calculate the daily dose for day 2, multiply the patient’s BSA times one hundred twenty-five micrograms per meter squared.
NARRATOR: On day 3, administer two hundred fifty micrograms per meter squared. To calculate the daily dose for day 3, multiply the patient’s BSA times two hundred fifty micrograms per meter squared.
NARRATOR: On day 4, administer 500 micrograms per meter squared. To calculate the daily dose for day 4, multiply the patient’s BSA times 500 micrograms per meter squared.
NARRATOR: The dose then remains the same on days 5 through 14. One thousand thirty micrograms per meter squared should be administered each day. To calculate the daily dose for days 5 through 14, multiply the patient’s BSA times one thousand thirty micrograms per meter squared.
NARRATOR: If a planned TZIELD infusion is missed, resume dosing by administering all remaining doses on consecutive days to complete the 14-day treatment course. Do not administer 2 doses on the same day.
NARRATOR: As per the body surface area-based dosing requirements, 2 vials of TZIELD per day may be needed for some individuals with a body surface area over one point nine four meters squared on days 5 through 14.
NURSE: For at least the first 5 days of the 14-day treatment course, administer premedication prior to the infusion.
NARRATOR: Premedication should consist of a nonsteroidal (non-stehr-OY-dahl) anti-inflammatory drug (or NSAID) (EN-sed) or acetaminophen (ah-see-tah-MIN-oh-fehn), an antihistamine (an-tee-HIS-tah-min), and/or an antiemetic (an-tee-eh-MI-tik). Administer additional doses of premedication if needed for symptom management at home and/or on additional treatment days.
NURSE: Before preparing to administer TZIELD, read the dosing and administration instructions within the prescribing information included in the carton.
NARRATOR: TZIELD is a clear and colorless solution supplied in 2-milligram per 2-milliliter single-dose vials.
NARRATOR: TZIELD vials must be kept refrigerated at 36 to 46 degrees Fahrenheit (or 2 to 8 degrees Celsius).
NARRATOR: TZIELD vials should be kept in their original packaging to protect from light and should be stored upright. Do not freeze or shake the vials.
NARRATOR: For product complaints, such as if the solution is not clear and colorless or if the vial shows any damage, call 1-800-633-1610.
NARRATOR: Before preparation, ensure you have all necessary supplies for the TZIELD infusion on hand: A vial of TZIELD (Remember, some patients may need 2 vials based on BSA).
NARRATOR: An empty sterile glass vial or polyvinyl chloride (or PVC) (pah-lee-VYE-nil KLOHR-eyed or P-V-C) infusion bag.
NARRATOR: 18 milliliters of zero point nine percent sodium chloride injection solution for use as a sterile diluent. These 18 milliliters can come from a variety of places, such as prefilled syringes or from drawing out 18 milliliters of solution from a sodium chloride injection solution bag. Use a new needle each time in order to follow sterile procedure.
NARRATOR: Syringes of various sizes. 18-gauge needles will be used to withdraw the zero point nine percent sodium chloride and the TZIELD solutions. Be sure to use a new needle with each injection.
NARRATOR: 25-milliliter zero point nine percent sodium chloride injection PVC infusion bag.
NARRATOR: IV catheter, routine infusion supplies, such as needles, gauze, tape, alcohol wipes, and a tourniquet. Note: Only use IV infusion bags made of polyvinyl chloride (PVC).
NARRATOR: IV access should be obtained before the drug is mixed, as to ensure completion within the allotted time window.
NURSE: Now, let’s get started!
NURSE: TZIELD requires dilution prior to administration. Be sure to inspect TZIELD visually before use. The supplied solution should be clear and colorless. Do not use TZIELD if particulate matter or coloration is seen.
NARRATOR: Prepare TZIELD in a 2-step dilution process using aseptic technique. Each vial is intended for a single dose only.
NARRATOR: Prepare a sterile glass vial with 18 milliliters of zero point nine percent sodium chloride injection solution or PVC infusion bag with 18 milliliters of zero point nine percent sodium chloride injection solution.
NARRATOR: For the first step in the dilution process, remove 2 milliliters of TZIELD from the vial...
NARRATOR: ...and slowly add it to the 18 milliliters of zero point nine percent sodium chloride injection solution.
NARRATOR: Mix gently by slowly inverting the vial or rocking the infusion bag to ensure that the solution mixes sufficiently.
NARRATOR: Remember, as per the body surface area-based dosing requirements, 2 vials of TZIELD per day may be needed for some individuals with a body surface area over one point nine four meters squared on days 5 through 14.
NARRATOR: The resulting 20-milliliter diluted solution now contains 100 micrograms per milliliter of TZIELD.
NARRATOR: Using an appropriately-sized syringe based on the volume of the calculated dose, withdraw the volume of diluted TZIELD solution required for that day’s dose.
NARRATOR: Refer to the recommended body surface area-based dosing regimen for TZIELD to calculate the recommended dosage for each treatment day based on the patient’s calculated BSA.
NARRATOR: Next, slowly add the contents of the syringe containing the patient- and treatment day-specific TZIELD dose to a 25-milliliter, zero point nine percent sodium chloride injection PVC infusion bag.
NARRATOR: Gently rock the infusion bag to ensure that the solution mixes sufficiently. Do not shake.
NARRATOR: If 2 vials are required for days 5 through 14 due to the BSA-based dosing requirements, 2 dilution solutions should be made, and the cumulative volume for the calculated dose added to a single infusion bag so that the entire dose for that day is contained in 1 infusion bag. Discard the unused portion of the remaining diluted TZIELD solution in the sterile glass vial or PVC infusion bag.
NARRATOR: Start the TZIELD infusion within 2 hours of preparation.
NARRATOR: If not using immediately, store the solution at room temperature (59 to 86 degrees Fahrenheit). The entire infusion will need to be completed within 4 hours of the start of preparation.
NARRATOR: An infusion pump is typically used to administer infusions. Infuse over a minimum of 30 minutes. You can slow down the infusion, if needed. Do not administer as an IV push or bolus (BOH-luhs) injection.
NARRATOR: Discard the infusion solution if not administered within 4 hours of the start of preparation.
NARRATOR: Remember that TZIELD is administered once daily for 14 consecutive days. The BSA-based dose increases each day from days 1 through 5, then remains the same for days 5 through 14.
NARRATOR: TZIELD prescribing information does not state that infusion needs to be administered at the same time on each treatment day. Two doses of TZIELD should not be administered on the same day.
NARRATOR: The most common adverse reactions were lymphopenia (lim-foh-PEE-nee-ah), rash, leukopenia (loo-koh-PEE-nee-ah) and headache. Throughout the course of treatment, monitor for cytokine (SY-toh-kyn) release syndrome (or CRS). Premedicate with antipyretics, antihistamines, and/or antiemetics prior to TZIELD treatment for the first 5 days and as needed thereafter.
NARRATOR: Monitor liver enzymes during treatment. Discontinue TZIELD treatment in patients who develop elevated ALT or AST more than 5 times the upper limit of normal or bilirubin more than 3 times the upper limit of normal.
NARRATOR: Treat symptoms of CRS with antipyretics, antihistamines, and/or antiemetics. If severe CRS develops, consider temporarily pausing dosing for 1 to 2 days (and administer the remaining doses to complete the full 14-day course on consecutive days) or discontinuing treatment.
NARRATOR: Serious infections. Monitor patients for signs and symptoms of infection during and after TZIELD treatment. If serious infection develops, treat appropriately, and discontinue TZIELD.
NARRATOR: Lymphopenia. Monitor white blood cell counts during the treatment period. If prolonged severe lymphopenia (less than 500 cells per microliter lasting 1 week or longer) develops, discontinue TZIELD.
NARRATOR: Hypersensitivity reactions. If severe hypersensitivity reactions occur, discontinue use of TZIELD and treat promptly.
NARRATOR: It is at the discretion of the treating healthcare provider to determine the frequency of additional labs to monitor during treatment based on clinical course and provider judgment.
NARRATOR: To report suspected Adverse Reactions, contact Provention Bio at 1-800-633-1610 or the FDA at 1-800-FDA-1088 or fda.gov/medwatch.
NARRATOR: Once the infusion solution has been completely administered, infuse an additional volume of zero point nine percent sodium chloride solution equal to the volume contained in the infusion tubing at the same constant rate to ensure that all the medication has been given.
NARRATOR: And that’s it. You’re all done! Post-infusion observation should be based on healthcare provider judgment. Patients should direct any questions to their healthcare provider.
NARRATOR: To learn more about TZIELD, visit TZIELDHCP.com.
NARRATOR: Hypersensitivity Reactions: Acute hypersensitivity reactions including serum sickness, angioedema (an-jee-oh-eh-DEE-mah), urticaria (er-teh-CAH-ree-ah), rash, vomiting and bronchospasm (BRAHN-koh-spaz-em) occurred in TZIELD-treated patients. If severe hypersensitivity reactions occur, discontinue TZIELD and treat promptly. Vaccinations: The safety of immunization with live-attenuated (live) vaccines with TZIELD-treated patients has not been studied. TZIELD may interfere with immune response to vaccination and decrease vaccine efficacy. Administer all age-appropriate vaccinations prior to starting TZIELD. Administer live vaccines at least 8 weeks prior to treatment. Live vaccines are not recommended during treatment, or up to 52 weeks after treatment. Administer inactivated (killed) vaccines or mRNA vaccines at least 2 weeks prior to treatment. Inactivated vaccines are not recommended during treatment or 6 weeks after completion of treatment.
NARRATOR: Adverse Reactions: Most common adverse reactions (greater than 10 percent) were lymphopenia, rash, leukopenia, and headache. Use in specific populations. Pregnancy: TZIELD may cause fetal harm. Lactation: A lactating woman may consider pumping and discarding breast milk during and for 20 days after TZIELD administration. Before prescribing TZIELD, please see full prescribing information, including patient selection criteria and medication guide.
Prior to initiation1
ALT=alanine aminotransferase; AST=aspartate aminotransferase; CMV=cytomegalovirus; EBV=Epstein–Barr virus; mRNA=messenger ribonucleic acid; NSAID=nonsteroidal anti-inflammatory drug; ULN=upper limit of normal.
See the clinical data for TZIELD in patients with Stage 2 T1D
Important Safety Information Anchor
INDICATION
TZIELD is a CD3-directed monoclonal antibody indicated to delay the onset of Stage 3 type 1 diabetes (T1D) in adults and pediatric patients aged 8 years and older with Stage 2 T1D.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
- Cytokine Release Syndrome (CRS): CRS occurred in TZIELD-treated patients during the treatment period and through 28 days after the last drug administration. Prior to TZIELD treatment, premedicate with antipyretics, antihistamines and/or antiemetics, and treat similarly if symptoms occur during treatment. If severe CRS develops, consider pausing dosing for 1 day to 2 days and administering the remaining doses to complete the full 14-day course on consecutive days; or discontinue treatment. Monitor liver enzymes during treatment. Discontinue TZIELD treatment in patients who develop elevated alanine aminotransferase or aspartate aminotransferase more than 5 times the upper limit of normal (ULN) or bilirubin more than 3 times ULN.
- Serious Infections: Use of TZIELD is not recommended in patients with active serious infection or chronic infection other than localized skin infections. Monitor patients for signs and symptoms of infection during and after TZIELD administration. If serious infection develops, treat appropriately, and discontinue TZIELD.
- Lymphopenia: Lymphopenia occurred in most TZIELD-treated patients. For most patients, lymphocyte levels began to recover after the fifth day of treatment and returned to pretreatment values within two weeks after treatment completion and without dose interruption. Monitor white blood cell counts during the treatment period. If prolonged severe lymphopenia develops (<500 cells per mcL lasting 1 week or longer), discontinue TZIELD.
- Hypersensitivity Reactions: Acute hypersensitivity reactions including serum sickness, angioedema, urticaria, rash, vomiting and bronchospasm occurred in TZIELD-treated patients. If severe hypersensitivity reactions occur, discontinue TZIELD and treat promptly.
- Vaccinations: The safety of immunization with live-attenuated (live) vaccines with TZIELD-treated patients has not been studied. TZIELD may interfere with immune response to vaccination and decrease vaccine efficacy. Administer all age-appropriate vaccinations prior to starting TZIELD.
- Administer live vaccines at least 8 weeks prior to treatment. Live vaccines are not recommended during treatment, or up to 52 weeks after treatment.
- Administer inactivated (killed) vaccines or mRNA vaccines at least 2 weeks prior to treatment. Inactivated vaccines are not recommended during treatment or 6 weeks after completion of treatment.
ADVERSE REACTIONS
Most common adverse reactions (>10%) were lymphopenia, rash, leukopenia, and headache.
USE IN SPECIFIC POPULATIONS
- Pregnancy: May cause fetal harm.
- Lactation: A lactating woman may consider pumping and discarding breast milk during and for 20 days after TZIELD administration.
Please see full Prescribing Information, including patient selection criteria, and Medication Guide. View Important Safety Information page.
REFERENCES
- TZIELD Prescribing Information. Provention Bio, Inc; 2023.
- Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098.
INDICATION
IMPORTANT SAFETY INFORMATION
INDICATION
TZIELD is a CD3-directed monoclonal antibody indicated to delay the onset of Stage 3 type 1 diabetes (T1D) in adults and pediatric patients aged 8 years and older with Stage 2 T1D.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
- Cytokine Release Syndrome (CRS): CRS occurred in TZIELD-treated patients during the treatment period and through 28 days after the last drug administration. Prior to TZIELD treatment, premedicate with antipyretics, antihistamines and/or antiemetics, and treat similarly if symptoms occur during treatment. If severe CRS develops, consider pausing dosing for 1 day to 2 days and administering the remaining doses to complete the full 14-day course on consecutive days; or discontinue treatment. Monitor liver enzymes during treatment. Discontinue TZIELD treatment in patients who develop elevated alanine aminotransferase or aspartate aminotransferase more than 5 times the upper limit of normal (ULN) or bilirubin more than 3 times ULN.
- Serious Infections: Use of TZIELD is not recommended in patients with active serious infection or chronic infection other than localized skin infections. Monitor patients for signs and symptoms of infection during and after TZIELD administration. If serious infection develops, treat appropriately, and discontinue TZIELD.
- Lymphopenia: Lymphopenia occurred in most TZIELD-treated patients. For most patients, lymphocyte levels began to recover after the fifth day of treatment and returned to pretreatment values within two weeks after treatment completion and without dose interruption. Monitor white blood cell counts during the treatment period. If prolonged severe lymphopenia develops (<500 cells per mcL lasting 1 week or longer), discontinue TZIELD.
- Hypersensitivity Reactions: Acute hypersensitivity reactions including serum sickness, angioedema, urticaria, rash, vomiting and bronchospasm occurred in TZIELD-treated patients. If severe hypersensitivity reactions occur, discontinue TZIELD and treat promptly.
- Vaccinations: The safety of immunization with live-attenuated (live) vaccines with TZIELD-treated patients has not been studied. TZIELD may interfere with immune response to vaccination and decrease vaccine efficacy. Administer all age-appropriate vaccinations prior to starting TZIELD.
- Administer live vaccines at least 8 weeks prior to treatment. Live vaccines are not recommended during treatment, or up to 52 weeks after treatment.
- Administer inactivated (killed) vaccines or mRNA vaccines at least 2 weeks prior to treatment. Inactivated vaccines are not recommended during treatment or 6 weeks after completion of treatment.
ADVERSE REACTIONS
Most common adverse reactions (>10%) were lymphopenia, rash, leukopenia, and headache.
USE IN SPECIFIC POPULATIONS
- Pregnancy: May cause fetal harm.
- Lactation: A lactating woman may consider pumping and discarding breast milk during and for 20 days after TZIELD administration.
Please see full Prescribing Information, including patient selection criteria, and Medication Guide. View Important Safety Information page.

