Efficacy and safety in Stage 2 T1D
Intervene with TZIELD, the only immunomodulator indicated for Stage 2 T1D in adults and pediatric patients aged 8 years and older.1


TRIALNET-10 (TN-10) STUDY DESIGN
A phase 2, randomized, double-blind, event-driven, placebo-controlled study in 76 patients, 8-49 years of age with Stage 2 T1D.2*
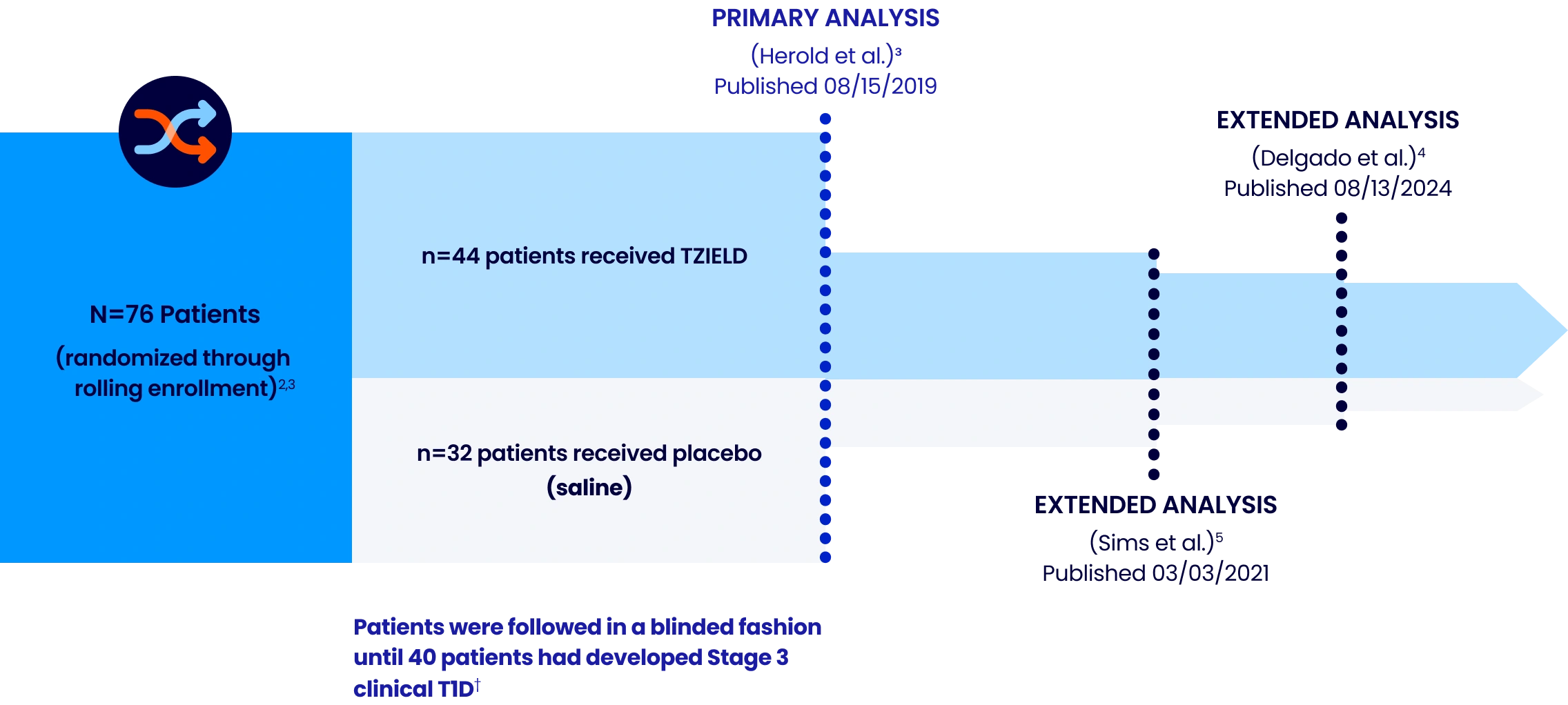
Extended analysis limitations: These extended analyses are not contained in the Prescribing Information. The TN-10 study was relatively small at the start of the study, and patient numbers decreased throughout the follow-up analyses. Therefore, definitive conclusions cannot be derived from these data.2,5
Patient results may vary.
*Stage 2 is defined as having 2 or more pancreatic islet AAbs (GADA, IAA, ICA, IA-2A, and ZnT8A) and dysglycemia on OGTT.²
†The primary efficacy endpoint was the time from randomization to diagnosis of Stage 3 clinical T1D. Criteria for Stage 3 clinical T1D onset were based on glucose testing or the presence of unequivocal hyperglycemia with acute metabolic decompensation, such as diabetic ketoacidosis.⁶
AAbs=autoantibodies; GADA=glutamic acid decarboxylase 65 AAb; IAA=insulin AAb; IA-2A=insulinoma-associated antigen 2 AAb; ICA=islet cell AAb; OGTT=oral glucose tolerance test; T1D=type 1 diabetes; ZnT8A=zinc transporter-8 AAb.
IN PATIENTS 8 YEARS AND OLDER WITH STAGE 2 T1D
For the first time ever, the onset of Stage 3 T1D can be delayed2,3
In the TN-10 study2,3:


more time
in presymptomatic Stage 2 vs placebo
Median time to Stage 3 onset:
TZIELD: 50 months
N=44
Placebo: 25 months
N=32
(HR 0.41; 95% CI, 0.22-0.78; P=0.0066 by an adjusted Cox proportional-hazards model stratified by age and OGTT status at randomization.)2,3
In patients with Stage 2 T1D, the median follow-up time was 51 months (range: 74 days to 2683 days).2,3*
Patients results may vary.
*Median follow-up time from the TZIELD Prescribing Information was calculated using the reverse Kaplan–Meier method.2,3
CI=confidence interval; HR=hazard ratio.
PRIMARY AND EXTENDED ANALYSIS
Median time to Stage 3 T1D onset across TN-10 analyses
![4.2 years with TZIELD® (teplizumab-mzwv) [50 months; N=44]. 2.1 years with placebo (25 months; N=32). 4.4 years with TZIELD® (teplizumab-mzwv) [52.2 months; N=44]; 2.3 years with placebo (27.3 months; N=32) [16 TZIELD® and 4 placebo patients remain in Stage 2]](/images/wp-content/uploads/2026/01/image-1-1-1024x590-1.webp)
![4.2 years with TZIELD® (teplizumab-mzwv) [50 months; N=44]. 2.1 years with placebo (25 months; N=32). 4.4 years with TZIELD® (teplizumab-mzwv) [52.2 months; N=44]; 2.3 years with placebo (27.3 months; N=32) [16 TZIELD® and 4 placebo patients remain in Stage 2]](/images/wp-content/uploads/2026/01/image-1-s-1-455x1024-1.webp)
Extended analysis limitations: This extended analysis is not contained in the Prescribing Information. The TN-10 study was relatively small at the start of the study, and patient numbers decreased throughout the follow-up analyses. Therefore, definitive conclusions cannot be derived from these data.2,5
Patient results may vary.
*HR 0.41; 95% CI, 0.22-0.78; P=0.0066 by an adjusted Cox proportional-hazards model stratified by age and OGTT status at randomization. The median follow-up time was 51 months (range: 74 days to 2683 days), calculated using the reverse Kaplan-Meier method.2,3
†The median follow-up time was 80.46 months, calculated using time to T1D diagnosis, end of study participation, or administration cutoff (end of study).4
EXTENDED ANALYSIS
~4 out of 10 TZIELD-treated patients remained in Stage 2 beyond 5 years4,7*
(Delgado et al. extended analysis, as of data cutoff July 2023)
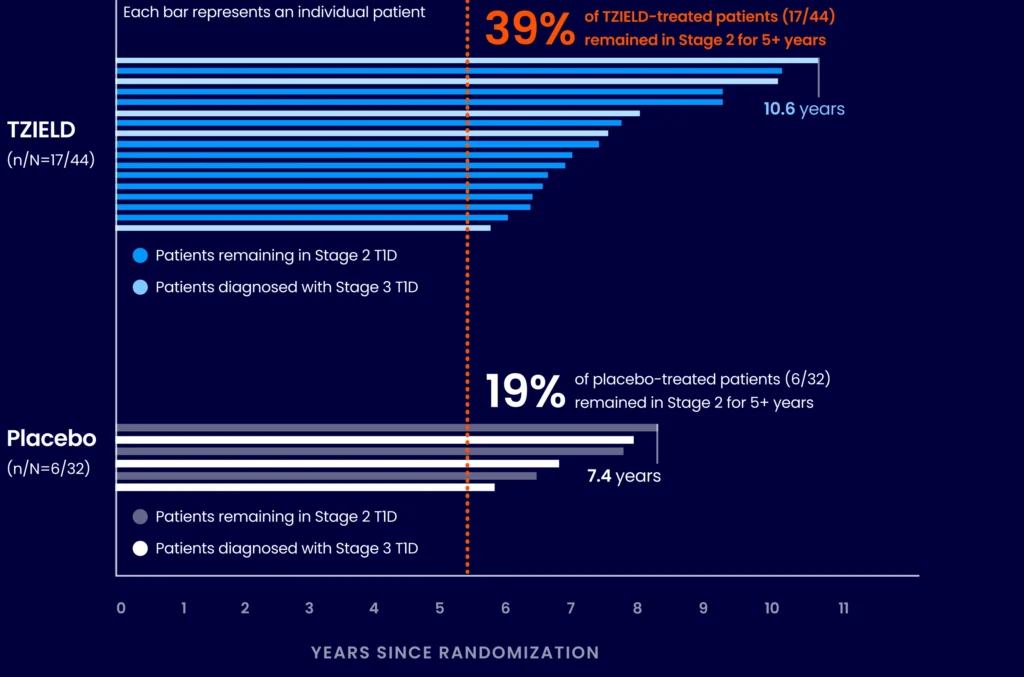
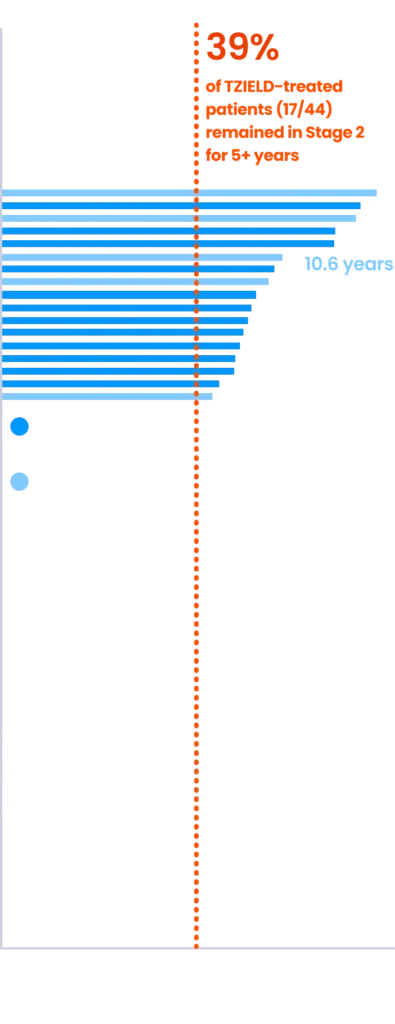
Extended analysis limitations: This extended analysis is not contained in the Prescribing Information. The TN-10 study was relatively small at the start of the study, and patient numbers decreased throughout the follow-up analyses. Therefore, definitive conclusions cannot be derived from these data.4,7
Patient results may vary.
*The median follow-up time was 80.46 months, calculated using time to TID diagnosis, end of study participation, or administration cutoff (end of study).4,7
TZIELD is an immunomodulator, not an immunosuppressant, with a well-established safety profile2*
Common adverse reactions in the TN-10 study†‡
| Adverse reaction | TZIELD (N=44) | Placebo (N=32) |
|---|---|---|
| Lymphopenia | 73% | 6% |
| Rash§ | 36% | 0% |
| Leukopenia | 21% | 0% |
| Headache | 11% | 6% |
| Neutropenia | 7% | 3% |
| Increased alanine aminotransferase | 5% | 3% |
| Nausea | 5% | 3% |
| Diarrhea | 5% | 0% |
| Nasopharyngitis | 5% | 0% |
The approval of TZIELD to treat patients with Stage 2 T1D was supported by a pooled safety analysis spanning 5 clinical studies including 773 patients.2
- 44 patients with Stage 2 T1D and 729 patients from unapproved populations||
*Adverse reactions in TZIELD-treated patients were also evaluated in a larger pool of adult and pediatric patients who participated in 5 controlled clinical studies (including the TN-10 study).2
†Adverse reactions that occurred in 2 or more TZIELD-treated patients.2
‡Adverse reactions that occurred during treatment and through 28 days after the last study drug administration.2
§Composite of rash-related terms, including rash erythematous, rash macular, rash papular, rash maculo-papular, and rash pruritic.2
||In these studies, 436 patients received a 14-day dosing regimen of TZIELD with total drug exposure comparable to the recommended approved dosage (Study TN-10), 168 patients received a 14-day course of TZIELD with a lower total TZIELD drug exposure, and 169 patients received a 6-day course of TZIELD with a lower total TZIELD drug exposure.2
Serious adverse reactions reported throughout the TN-10 study with greater frequency in TZIELD-treated patients vs placebo-treated patients2
| Adverse reaction | TZIELD (N=44) | Placebo (N=32) |
|---|---|---|
| Cytokine release syndrome (CRS) | 2% | 0% |
| Serious infections¶ | 9% | 0% |
| Hypersensitivity reactions and serum sickness | 2% | 0% |
| Lymphopenia | 73% | 6% |
| Neutropenia | 7% | 3% |
TZIELD has no black box warning or contraindications.
¶Serious infections included cellulitis, gastroenteritis, pneumonia, and wound infection any time during or after the first dose of study treatment.2
CYTOKINE RELEASE SYNDROME (CRS)
Of the 76 patients in the TN-10 study, CRS occurred in a single TZIELD-treated patient2
- Cytokine release in the context of TZIELD treatment is likely due to partial CD3 agonism and is usually seen during the initial days of infusion until the activated T cells become quiescent2,8,9
- The CRS experience by the single TZIELD-treated patient in TN-10 was a Grade 2 event, and the subject recovered and completed the entire 14-day treatment course without dose reduction10#
Mitigation guidance for CRS2
- CRS can be mitigated by premedicating with antipyretics, antihistamines and/or antiemetics, or by pausing dosing
- Premedicate, monitor liver enzymes, and discontinue treatment in those that develop elevated alanine aminotransferase or aspartate aminotransferase levels more than 5 times the upper limit of normal, and, if severe CRS develops, consider temporarily pausing dosing
#According to the National Cancer Institute Common Technology Criteria for Adverse Events (NCI-CTCAE), version 3.0.10
LYMPHOPENIA PREVALENCE IN TN-10
Lymphopenia is an expected and common adverse reaction to TZIELD that is often resolved by Week 62
Week 6: average lymphocyte counts return to baseline2,3**
Lymphopenia and
treatment with TZIELD2
- Average lymphocyte count nadir occurred at Day 5 of treatment, with recovery and return to baseline by Week 6 in most patients
- Lymphopenia occurred in the absence of T-cell depletion
- TZIELD was not commonly associated with the symptomatic reactivation of EBV or CMV††
Guidance for lymphopenia2:
- If lymphopenia occurs, monitor white blood cell counts during the 2-week treatment period
- If prolonged severe lymphopenia (<500 cells per mcL, lasting 1 week or longer) develops, discontinue TZIELD
**Adapted from Herold KC, et al. Means and confidence intervals are shown.
††One TZIELD-treated patient in the TN-10 study reported symptoms consistent with the reactivation of EBV.3
CMV=cytomegalovirus; EBV=Epstein–Barr virus.
Additional warnings and precautions

Serious infections2
Use of TZIELD is not recommended in patients with active serious infection or chronic infection. Monitor for signs and symptoms of infection during and after TZIELD treatment. If a serious infection develops, discontinue TZIELD.

Vaccinations2
Administer all age-appropriate vaccinations prior to starting TZIELD. See recommendations regarding live-attenuated, inactivated, and mRNA vaccines.

Hypersensitivity reactions2
If severe hypersensitivity reactions occur, discontinue TZIELD and treat promptly. Acute hypersensitivity reactions, including serum sickness, angioedema, urticaria, rash, vomiting and bronchospasm, occurred in TZIELD-treated patients.

Learn how to support your patients' treatment
Important Safety Information Anchor
INDICATION
TZIELD is a CD3-directed monoclonal antibody indicated to delay the onset of Stage 3 type 1 diabetes (T1D) in adults and pediatric patients aged 8 years and older with Stage 2 T1D.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
- Cytokine Release Syndrome (CRS): CRS occurred in TZIELD-treated patients during the treatment period and through 28 days after the last drug administration. Prior to TZIELD treatment, premedicate with antipyretics, antihistamines and/or antiemetics, and treat similarly if symptoms occur during treatment. If severe CRS develops, consider pausing dosing for 1 day to 2 days and administering the remaining doses to complete the full 14-day course on consecutive days; or discontinue treatment. Monitor liver enzymes during treatment. Discontinue TZIELD treatment in patients who develop elevated alanine aminotransferase or aspartate aminotransferase more than 5 times the upper limit of normal (ULN) or bilirubin more than 3 times ULN.
- Serious Infections: Use of TZIELD is not recommended in patients with active serious infection or chronic infection other than localized skin infections. Monitor patients for signs and symptoms of infection during and after TZIELD administration. If serious infection develops, treat appropriately, and discontinue TZIELD.
- Lymphopenia: Lymphopenia occurred in most TZIELD-treated patients. For most patients, lymphocyte levels began to recover after the fifth day of treatment and returned to pretreatment values within two weeks after treatment completion and without dose interruption. Monitor white blood cell counts during the treatment period. If prolonged severe lymphopenia develops (<500 cells per mcL lasting 1 week or longer), discontinue TZIELD.
- Hypersensitivity Reactions: Acute hypersensitivity reactions including serum sickness, angioedema, urticaria, rash, vomiting and bronchospasm occurred in TZIELD-treated patients. If severe hypersensitivity reactions occur, discontinue TZIELD and treat promptly.
- Vaccinations: The safety of immunization with live-attenuated (live) vaccines with TZIELD-treated patients has not been studied. TZIELD may interfere with immune response to vaccination and decrease vaccine efficacy. Administer all age-appropriate vaccinations prior to starting TZIELD.
- Administer live vaccines at least 8 weeks prior to treatment. Live vaccines are not recommended during treatment, or up to 52 weeks after treatment.
- Administer inactivated (killed) vaccines or mRNA vaccines at least 2 weeks prior to treatment. Inactivated vaccines are not recommended during treatment or 6 weeks after completion of treatment.
ADVERSE REACTIONS
Most common adverse reactions (>10%) were lymphopenia, rash, leukopenia, and headache.
USE IN SPECIFIC POPULATIONS
- Pregnancy: May cause fetal harm.
- Lactation: A lactating woman may consider pumping and discarding breast milk during and for 20 days after TZIELD administration.
Please see full Prescribing Information, including patient selection criteria, and Medication Guide. View Important Safety Information page.
REFERENCES
- TZIELD Prescribing Information. Provention Bio, Inc; 2023.
- diaTribe Learn. FDA approves Tzield (teplizumab) to delay type 1 diabetes. Published November 17, 2022. Updated November 21, 2022. https://diatribe.org/fda-approves-tzield-teplizumab-delay-type-1-diabetes
- Herold KC, Bundy BN, Long SA, et al; Type 1 Diabetes TrialNet Study Group. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med. 2019;381(7):603-613.
- Lledó-Delgado A, Preston-Hurlburt P, Currie S, et al. Teplizumab induces persistent changes in the antigen-specific repertoire in individuals at risk for type 1 diabetes. J Clin Invest. 2024;134(18):e177492.
- Sims EK, Bundy BN, Stier K, et al. Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals + Supplementary Appendix. Sci Transl Med. 2021;13(583):eabc8980.
- Department of Health & Human Services. FDA briefing document—endocrinologic and metabolic drugs advisory committee meeting: teplizumab BLA 761183. May 27, 2021. https://www.fda.gov/media/178821/download
- Data on file. DOF-BLA 761183-0003. Sanofi.
- Baumgart DC, Lowder JN, Targan S, Sandborn WJ, Frankel MB. Transient cytokine-induced liver injury following administration of the humanized anti-CD3 antibody visilizumab (HuM291) in Crohn’s Disease. Am J Gastroenterol. 2009;104(4):868-876.
- Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56.
- Data on file. DOF-TN-10-CSR. Sanofi.
INDICATION
IMPORTANT SAFETY INFORMATION
INDICATION
TZIELD is a CD3-directed monoclonal antibody indicated to delay the onset of Stage 3 type 1 diabetes (T1D) in adults and pediatric patients aged 8 years and older with Stage 2 T1D.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
- Cytokine Release Syndrome (CRS): CRS occurred in TZIELD-treated patients during the treatment period and through 28 days after the last drug administration. Prior to TZIELD treatment, premedicate with antipyretics, antihistamines and/or antiemetics, and treat similarly if symptoms occur during treatment. If severe CRS develops, consider pausing dosing for 1 day to 2 days and administering the remaining doses to complete the full 14-day course on consecutive days; or discontinue treatment. Monitor liver enzymes during treatment. Discontinue TZIELD treatment in patients who develop elevated alanine aminotransferase or aspartate aminotransferase more than 5 times the upper limit of normal (ULN) or bilirubin more than 3 times ULN.
- Serious Infections: Use of TZIELD is not recommended in patients with active serious infection or chronic infection other than localized skin infections. Monitor patients for signs and symptoms of infection during and after TZIELD administration. If serious infection develops, treat appropriately, and discontinue TZIELD.
- Lymphopenia: Lymphopenia occurred in most TZIELD-treated patients. For most patients, lymphocyte levels began to recover after the fifth day of treatment and returned to pretreatment values within two weeks after treatment completion and without dose interruption. Monitor white blood cell counts during the treatment period. If prolonged severe lymphopenia develops (<500 cells per mcL lasting 1 week or longer), discontinue TZIELD.
- Hypersensitivity Reactions: Acute hypersensitivity reactions including serum sickness, angioedema, urticaria, rash, vomiting and bronchospasm occurred in TZIELD-treated patients. If severe hypersensitivity reactions occur, discontinue TZIELD and treat promptly.
- Vaccinations: The safety of immunization with live-attenuated (live) vaccines with TZIELD-treated patients has not been studied. TZIELD may interfere with immune response to vaccination and decrease vaccine efficacy. Administer all age-appropriate vaccinations prior to starting TZIELD.
- Administer live vaccines at least 8 weeks prior to treatment. Live vaccines are not recommended during treatment, or up to 52 weeks after treatment.
- Administer inactivated (killed) vaccines or mRNA vaccines at least 2 weeks prior to treatment. Inactivated vaccines are not recommended during treatment or 6 weeks after completion of treatment.
ADVERSE REACTIONS
Most common adverse reactions (>10%) were lymphopenia, rash, leukopenia, and headache.
USE IN SPECIFIC POPULATIONS
- Pregnancy: May cause fetal harm.
- Lactation: A lactating woman may consider pumping and discarding breast milk during and for 20 days after TZIELD administration.
Please see full Prescribing Information, including patient selection criteria, and Medication Guide. View Important Safety Information page.



