Why to screen
Why screen
Screening can help identify at-risk patients before symptoms arise
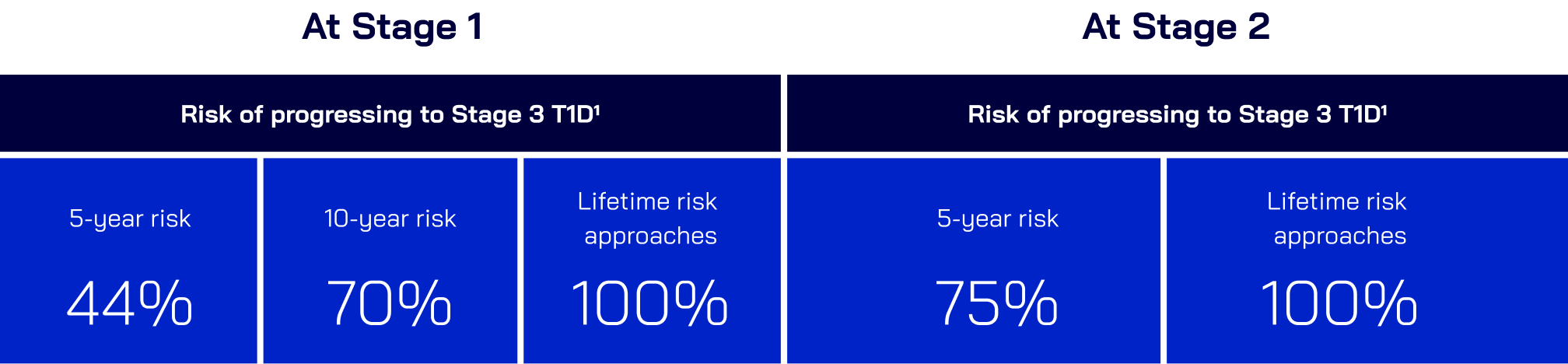
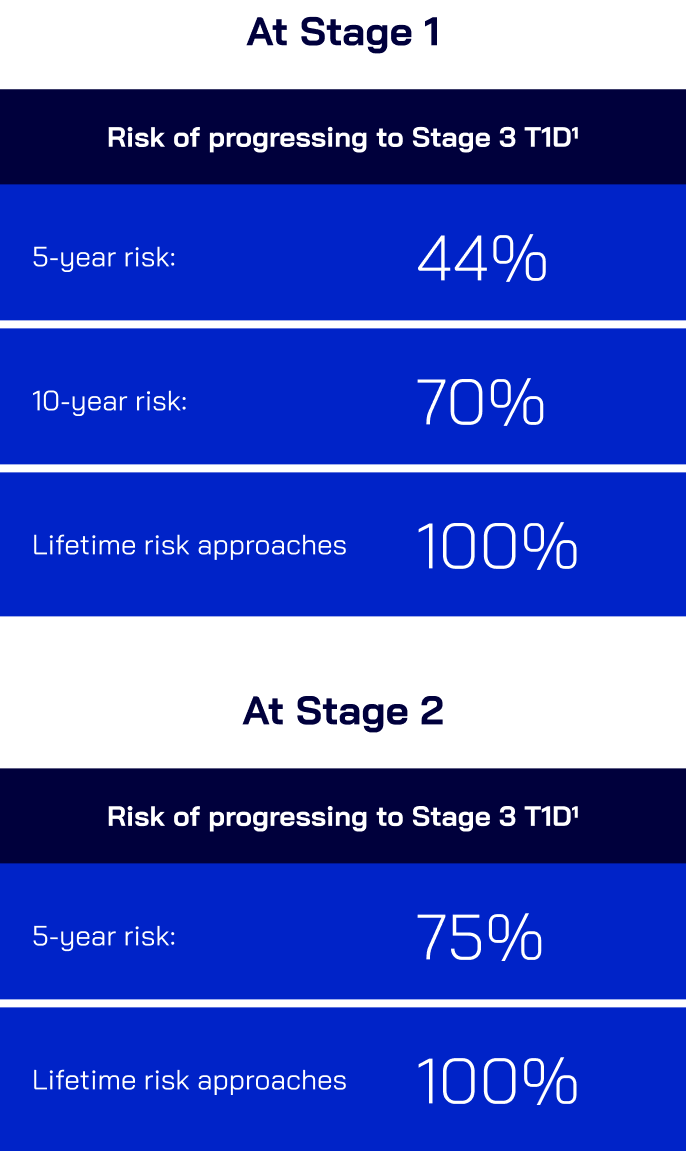
Once patients are in Stage 1 T1D,
progression to Stage 3 becomes inevitable
progression to Stage 3 becomes inevitable
Identifying autoimmune T1D early can give you more time to4:
Assemble a care team
Refer your patients to specialists in a number of areas, including mental health and other support areas.
Be vigilant
Advise patients and caregivers to be vigilant for symptoms of hyperglycemia and DKA.
Seek presymptomatic intervention
Talk to your patients and their care teams to know when and how to seek presymptomatic intervention.
Screen and stage for the opportunity to intervene before Stage 3 T1D
DKA=diabetic ketoacidosis; T1D=type 1 diabetes.
References: 1. Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38(10):1964-1974. 2. Elding Larsson H, Vehik K, Bell R, et al. Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care. 2011;34(11):2347-2352. 3. Barker JM, Goehrig SH, Barriga K, et al. DAISY study. Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care. 2004;27(6):1399-1404. 4. Scheiner G, Weiner S, Kruger D, Pettus J. Screening for type 1 diabetes: Role of the diabetes care and education specialist. ADCES Pract. 2022;10(5):20-25.